Subjects: PDT, TRI
J INTS BIO, AACR 2024 - 'JIN-A02', a Novel Oral 4th Generation EGFR TKI, showing Clinical Benefits in the Ongoing Phase 1 Study
SEOUL, South Korea, April 11, 2024 /PRNewswire/ -- J INTS BIO announced the results of its ongoing Phase 1 clinical study of JIN-A02, a 4th generation EGFR-TKI for NSCLC treatment, at the 8th April poster session of the 2024 American Cancer Research Society (AACR) Annual Meeting held in San Diego, California, from 5th to 10th April (U.S. local time).
'JIN-A02' is a 4th generation EGFR-TKI targeting C797S mutation that causes resistance to 3rd generation EGFR-TKI (such as Osimertinib, Lazertinib), commonly used in the treatment of NSCLC. 'JIN-A02' also targets T790M mutation independently of C797S mutation.
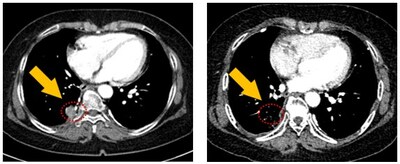
This ongoing Phase 1 clinical study is currently in its 4th Dose Level of 100mg daily and patients have showed increasing clinical benefits with each increasing dose levels. To-date, Partial Response (PR) was confirmed in one subject on the prior dose level of 50mg daily and stable diseases in two other subjects separately in Dose Level 50mg and 25mg daily.
Despite the initial low doses, JIN-A02 has showed clinical benefits immediately, cumulating in a Partial Response at the 3rd Dose Level of 50mg, which is an encouraging result in such an early stage of clinical development, the company explained. Furthermore, JIN-A02 has showed a favorable safety profile with no cardiotoxicity, rash or diarrhea reported, even at the current Dose Level of 100mg daily and the longest duration of treatment is over 8 months and still continuing, the company added. The 5th Dose Level of 150mg daily is expected to begin in 3Q 2024.
J INTS BIO confirmed the favorable safety and efficacy signals of 'JIN-A02' with clinical benefits in this poster presentation in San Diego and greater clinical impact is expected as the study progresses.
SOURCE J INTS BIO
These press releases may also interest you
|
News published on and distributed by: