Subject: RCL
Public advisory - Recall of one lot of JAMP Guanfacine XR 4 mg tablets due to contamination with foreign matter
OTTAWA, ON, April 12, 2024 /CNW/ -
- Product: JAMP-Guanfacine XR 4 mg tablets
- Issue: Health products ? Contamination
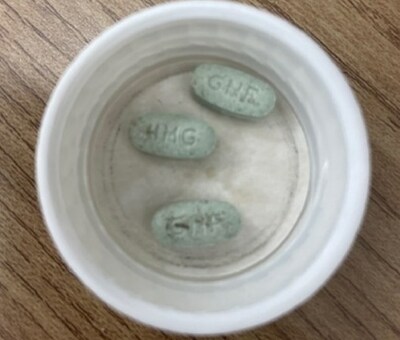
Health products ? Product safety - What to do: Check your or your child's pill bottle to ensure that the green JAMP Guanfacine XR 4mg tablets do not have any brown or amber-coloured stains. If stains are identified, or if you are unsure, return it to your pharmacy to obtain a replacement. Talk to a health care professional if you have any questions or any health concerns.
Product | DIN | Lot | Expiry |
JAMP-Guanfacine XR 4 mg tablets | 02523582 | GEF2013 | 2024-NOV |
Jamp Pharma Corporation is recalling one lot of JAMP Guanfacine extended release (XR) 4 mg tablets because some tablets may have been contaminated with foreign matter during manufacturing. The foreign matter causes brown or amber-coloured staining on the tablet. The foreign matter is composed of a combination of cellulose, lubricant oil, calcium, and/or iron oxide. These substances are not expected to pose any serious health risks; however, it is recommended that the medication be replaced if stains are identified.
JAMP Guanfacine XR is a prescription drug used to treat Attention Deficit Hyperactivity Disorder (ADHD) in children and adolescents 6 to 17 years of age. The 4 mg tablet is green, oval shaped, with "GNF" stamped on one side and "4MG" stamped on the other.
Health Canada is monitoring the company's recall and its implementation of any necessary corrective and preventative actions to stop this issue from reoccurring. The Department will inform the public if any new health risks are identified.
- Check your or your child's pill bottle to ensure that the green JAMP Guanfacine XR 4mg tablets do not have any brown or amber-coloured stains. If stains are identified, or if you are unsure, return it to your pharmacy for a replacement.
- Talk to a health care professional if you have questions or have health concerns.
- Contact JAMP Pharma Corporation by calling 450-449-4326 (option 3) or 1-866-399-9091 (option 3), or by email at [email protected] if you have questions about this recall.
- Report any health product-related side effects or complaints to Health Canada.
- Health care professionals, such as pharmacists, should check bottles of Guanfacine XR 4 mg tablets before dispensing and report any unusual tablets or other issue to the company and to Health Canada.
Également disponible en français
SOURCE Health Canada (HC)
These press releases may also interest you
|
News published on and distributed by: