Subjects: RCL, PSF, CFG
Advisory - Unauthorized drugs seized from Tokyo Beauty in Burnaby, B.C., may pose serious health risks
OTTAWA, ON, July 7, 2021 /CNW/ -
Summary
- Product: Multiple unauthorized drugs (including an acne gel, an antibiotic cream, eye drops and eyewashes) from Tokyo Beauty in Burnaby, B.C.
- Issue: Products are unauthorized and labelled to contain prescription drugs, which may pose serious health risks.
- What to do: Stop using these products. Consult your health care professional if you have used any of these products and have health concerns.
Issue
Health Canada has seized several health products?including an acne gel, an antibiotic cream, eye drops and eyewashes?from Tokyo Beauty in the Metropolis at Metrotown mall, Burnaby, B.C., because they are unauthorized drugs and may pose serious health risks.
According to the product labels, the products contain prescription drugs (see table below for details). Prescription drugs should only be taken under the advice and supervision of a health care professional because of the risk of interactions with other medications and side effects. Prescription drugs can only be legally sold with a prescription.
Selling unauthorized drugs is illegal in Canada. Unauthorized drugs have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality and may pose serious health risks. They may contain ingredients, additives or contaminated ingredients not listed on the label. In addition, they may lack the active ingredients Canadians would expect them to contain to help maintain and improve their health. For all of these reasons, unauthorized health products could cause serious health effects.
All of the unauthorized health products that Health Canada seized are packaged and labelled in Japanese characters. As a result, information about ingredients, usage, dosage and side effects may not be understood by all consumers.
Health Canada has previously seized unauthorized products from another Tokyo Beauty store with the same owner, located in Richmond, B.C., and issued advisories in March 2021 and February 2019. The Department will continue to take action to stop this illegal activity and update Canadians as needed.
Affected products
Photo | Product | Risk |
Dalacin T Gel 1% Antibacterial gel for acne | Labelled (in Japanese) to contain clindamycin
| |
Gentashin 0.1% Antibiotic Cream Antibiotic cream | Labelled (in Japanese) to contain gentamicin | |
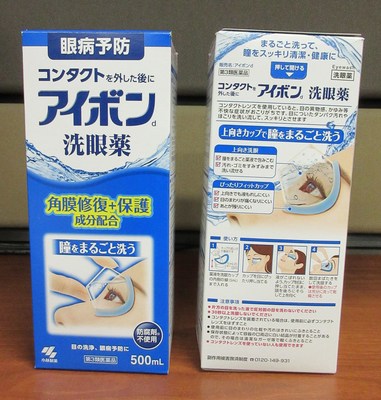
Kobayashi Aibon/Eyebon Eyewash (blue) Eyewash | Labelled (in Japanese) to contain aminocaproic acid | |
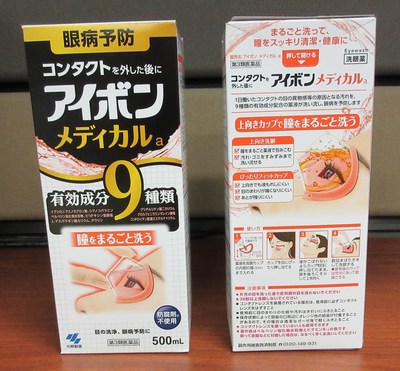
Kobayashi Aibon/Eyebon Eyewash Medicala (black) Eyewash | Labelled (in Japanese) to contain aminocaproic acid | |
Santen Sante Beautéye Contact Eye drops | Labelled (in Japanese) to contain neostigmine methylsulfate | |
Santen Sante FX Neo (blue) Eye drops | Labelled (in Japanese) to contain neostigmine methylsulfate and aminocaproic acid
| |
Santen Sante FX Neo (silver) Eye drops | Labelled (in English) to contain neostigmine methylsulfate and aminocaproic acid | |
Santen Sante PC Eye drops | Labelled (in Japanese) to contain neostigmine methylsulfate |
What you should do
- Stop using these products. Consult your health care professional if you have used any of these products and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database or Licensed Natural Health Products Database.
- Report any health product-related side effects or complaints to Health Canada.
Background
Aminocaproic acid: This is a prescription drug ingredient used to decrease bleeding in various clinical situations. Exposure to aminocaproic acid in the eye may affect the eye itself, and the acid may be absorbed through the tear ducts into the blood. Side effects may include watery eyes, vision changes, headache, dizziness, nausea, muscle weakness and skin rash.
Clindamycin: In topical format (i.e., applied to the skin), this is a prescription antibiotic approved in Canada to treat bacterial infections, including those associated with acne. The product should not be used in individuals with a history of ulcerative colitis (inflamed bowel) or a history of inflamed bowel associated with antibiotic use (antibiotic-associated colitis). Side effects may include dry or scaly skin, peeling skin, a stinging or burning feeling on the skin, eye pain, itching, hives, redness and gastrointestinal symptoms (such as indigestion and gas). Safety and effectiveness in children under the age of 12 has not been established.
Gentamicin is a prescription antibiotic drug applied topically (to the skin) to treat minor skin infections. It can cause skin redness and irritation. Prolonged use can cause fungal or bacterial infections. Gentamicin may be absorbed into the blood if applied to large areas of skin, especially if the areas are cracked or raw, which increases the risk of serious side effects. Serious side effects include allergic reactions (e.g., rash, hives, and red, swollen, blistered, or peeling skin with or without fever, trouble breathing, swelling of the mouth, face, lips, tongue or throat) and kidney and hearing damage. It should not be used in patients who are allergic to gentamicin. Gentamicin may interact with other drugs and should be used with caution with other antibiotics, anti-cancer drugs, diuretics, immunosuppressants, and nonsteroidal anti-inflammatories.
Neostigmine methylsulfate: There are no approved eye drops containing neostigmine methylsulfate on the Canadian market. In the past, drugs similar to neostigmine were used to treat glaucoma. These medications are no longer widely used because of the significant number of potential eye-related side effects, including blurred distance vision, frontal headaches, twitching lids, red eyes, cataracts, allergic reactions, iris cysts, retinal detachment and the potential for causing a specific type of glaucoma attack. In addition, absorption into the nose via the tear duct may cause serious cardiac and respiratory side effects.
Également disponible en français
SOURCE Health Canada
These press releases may also interest you
|
News published on and distributed by: