Subjects: RCL, PSF
Voluntary Nationwide Recall: One Lot of Mirvalatm 28 (desogestrel and Ethinyl Estradiol Tablets, Usp) 0.150 Mg / 0.030 Mg Due to Possibility of One Placebo Pill in Place of An Active Pill
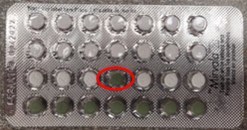
TORONTO, Sept. 30, 2021 /CNW/ - Apotex Inc. is conducting a voluntary nationwide recall of one lot of Mirvalatm 28 (Desogestrel and Ethinyl Estradiol Tablets, USP) from wholesalers, distributors, retailers, pharmacies, and consumers. The identified lot is being recalled out of an abundance of caution due to a highly detectable defect of one placebo pill (green) in place of a birth control pill (white). This product is manufactured by a 3rd party source, Laboratorios LEON FARMA, S.A. Spain.
The affected lot can be identified by following the information on the product carton and/or blister card:
DIN | UPC Code | Lot Number | Expiry Date |
02410257 | 771313225328 | LF21272B | 08/2022 |
Mirvalatm 28 (Desogestrel and Ethinyl Estradiol Tablets, USP 0.150 mg / 0.030 mg) is indicated for conception control. For further details, please refer to the package insert. The product is packaged as 28 tablets in a blister packet.
Patients can refer to both the Patient Information Leaflet and Product Monograph of Mirvalatm 28 (Desogestrel and Ethinyl Estradiol Tablets, USP 0.150 mg / 0.030 mg) which provide clear instruction for the management of missed tablets or missed dose. To date, there are no adverse event reports received for lot LF21272B.
The impacted lot was distributed nationwide in Canada to wholesalers, distributors and pharmacies by Apotex between December 08, 2020 and August 24, 2021. Apotex is currently notifying its affected direct account customers by sending the recall notification letter and is arranging for return of the impacted product lot.
Patients who have received the impacted lot of Mirvalatm 28 (Desogestrel and Ethinyl Estradiol Tablets, USP 0.150 mg / 0.030 mg) should return the product to a pharmacy for replacement. Additionally, patients/consumers may contact Apotex Inc. via Stericycle by calling at 1-866-534-9986 or by email [email protected] if they have any questions about the recall.
To report a suspected adverse reaction associated with the use of Mirvalatm 28 (Desogestrel and Ethinyl Estradiol Tablets, USP 0.150 mg / 0.030 mg) patients may contact Apotex by calling 1-800-667-4708 or 416-401-7780 (follow prompts), by email at [email protected] or by fax at 1-866-429-9133 or 416-401-3819.
Patients may also report any suspected adverse reactions associated with the use of health products to Health Canada by calling toll free at 1-866-234-2345 or by visiting MedEffect Canada's Web page on Adverse Reaction Reporting https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada.html.
Incorrect packaging configuration
Correct packaging configuration:
About Apotex
Apotex Inc. is a proudly Canadian, global pharmaceutical company that produces high-quality, affordable medicines for patients around the world. Apotex employs almost 8,000 people worldwide in manufacturing, R&D, and commercial operations. Apotex Inc. exports to more than 100 countries and territories and operates in more than 45 countries, with a significant presence in Canada, the US, Mexico, and India. Through vertical integration, Apotex is comprised of multiple divisions and affiliates including Apotex Inc., focused on generics; Apobiologix, a division of Apotex Inc. focused on biosimilar development; Aveva, an affiliate of Apotex Inc. fully integrated global developer and manufacturer of complete transdermal solutions; Apotex Consumer Products, a division of Apotex Inc. focused on brand name products; and Global Active Pharmaceutical Ingredients (GAPI), a division of Apotex Inc. focused on the manufacturing of active pharmaceutical ingredients (API) for Apotex and third parties. For more information visit: www.apotex.com.
SOURCE Apotex Inc.
These press releases may also interest you
|
News published on and distributed by: