Subjects: RCL, PSF, CFG
Public Advisory - Counterfeit Nuceiva injectable drug seized from New You Spa in Vaughan, Ontario
OTTAWA, ON, Sept. 6, 2022 /CNW/ -
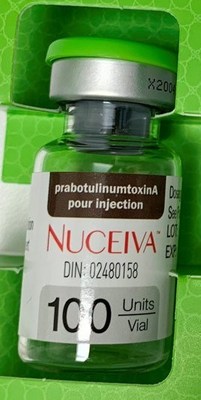
- Product: Counterfeit Nuceiva (Prabotulinumtoxin A) for injection
- Issue: Health products - Product safety, Unauthorized product
- What to do: Do not use this counterfeit product. Consult with a health care professional if you think you may have been administered this product at this location and you have health concerns. Report any health product-related adverse reactions or complaints to Health Canada.
Product | Strength | DIN | Lot | Expiry date |
Counterfeit Nuceiva (Prabotulinumtoxin A) for injection | 100 units/vial | 02480158 | 121661 | 2023-04 |
Health Canada inspectors seized counterfeit Nuceiva (Prabotulinumtoxin A) from a New You Spa location in Vaughan, Ontario (Unit 5, 60 Winges Road). Nuceiva is botulinum toxin type A prescription drug that is administered by injection for the temporary improvement of facial wrinkling in adults 18 years of age and older.
Health Canada has confirmed with Evolus Inc., the manufacturer of Nuceiva, that the seized products are counterfeit. The counterfeit Nuceiva is labelled with lot 121661 and expiry 2023-04. Evolus Inc. confirmed there is an authorized lot with the same number; the lot expired in 2022-04. In addition, Evolus Inc. confirmed that:
- the counterfeit carton is missing the manufacturer (Evolus Inc.) and distributor (Clarion Medical Technologies Inc.) information;
- the inlay in the counterfeit carton is green, whereas the inlay in an authentic Nuceiva carton is white;
- the vial label is missing the manufacturer name; and
- the vial cap is a different colour. Authentic Nuceiva has a grey cap, rather than the green or white cap found on the counterfeit product.
Counterfeit drugs are made to look like authentic products, but they may not be the same and they can pose serious health risks. For example, they could contain a higher dosage than shown on the label, contaminants or hidden dangerous ingredients that can seriously harm your health. On the other hand, they might not contain the drug at all. Unlike authorized and authentic drug products, counterfeit drugs have not been assessed by Health Canada for safety, effectiveness and quality. Selling counterfeit health products in Canada is illegal.
Botulinum toxin type A is used for cosmetic purposes to treat facial wrinkling. Some products containing botulinum toxin type A are also used to treat severe muscle spasms in the neck, eye and foot, as well as chronic migraines, urinary incontinence, and excessive sweating. Authorized botulinum toxin type A products should only be used under specialist supervision and only if the benefits of treatment are considered to outweigh the risks. Potential risks associated with injecting an unauthorized Botulinum toxin type A product can range from mild local paralysis to death. In addition, unauthorized injectable health products for cosmetic purposes carry significant risk because of the potential for infection, scarring and poor outcomes. All products administered by injection in Canada must be authorized for sale by Health Canada.
Également disponible en français
SOURCE Health Canada
These press releases may also interest you
|
News published on and distributed by: