Subjects: RCL, CFG
Public advisory - Pediatrix Acetaminophen Oral Solution for children: One lot recalled due to potential risk of overdose
OTTAWA, ON, Jan. 17, 2024 /CNW/ -
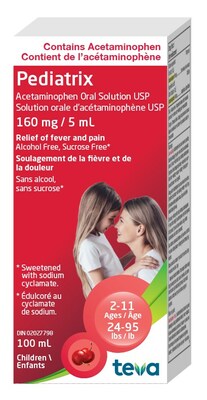
- Product: Pediatrix Acetaminophen Oral Solution, 160mg/5mL
- Issue: Health products ? Product safety
- What to do: Stop using the affected product. If your child shows sign/symptoms of acetaminophen overdose (as specified below), call your local poison control centre or emergency health care services immediately. Consult a health care professional if you have questions or concerns about your child's health.
Product | DIN | Lot | Expiry |
Pediatrix (Acetaminophen Oral Solution), 160mg/5mL | 02027798 | MC0079 | Aug 2025 |
Teva Canada Ltd. is recalling one lot of Pediatrix Acetaminophen Oral Solution after routine product testing found a higher than acceptable amount of acetaminophen in the affected lot (approximately 185mg/5mL rather than the approved and labelled 160mg/5mL). This could lead to children receiving too much acetaminophen. Children may be especially at risk of the effects of acetaminophen overdose given their small size and developing bodies.
Signs of acetaminophen overdose include nausea, vomiting, lethargy, sweating, loss of appetite and pain in the upper part of the abdomen or stomach. Abdominal pain may be the first sign of liver damage and may not be apparent for 24 to 48 hours. Liver damage may result in liver failure or, in the most severe cases, death.
The product is available without a prescription and is used to relieve mild to moderate pain and fever in children from 2 to 11 years of age.
Health Canada is monitoring the company's recall and its implementation of any necessary corrective and preventative actions. It will inform the public if any new health risks are identified.
Given the limited scope of the recall, it will not have any impact on the general availability of children's acetaminophen products.
- Verify the lot number on your medication. The lot number on a medication bottle is commonly found next to the expiration date.
- Stop using the affected product. If your child shows signs/symptoms of acetaminophen overdose, such as nausea, vomiting, lethargy, sweating, loss of appetite and pain in the upper part of the abdomen or stomach, call your local poison control centre or emergency health care services immediately. Consult a health care professional if you have questions or concerns about your child's health.
- Return the product to your local pharmacy for proper disposal. Contact Teva Canada Customer Care by calling 1-800-268-4129, or emailing [email protected], if you have questions about the recall.
- Report any health product-related side effects or complaints to Health Canada.
Également disponible en français
SOURCE Health Canada (HC)
These press releases may also interest you
|
News published on and distributed by: